your cryogenic
storage

MVE HE 800C Series
For compact, high-performance cryogenic storage

MVE HEco™
Looking for extended hold times and unmatched efficiency? The MVE HEco™ Series has you covered.

Racking solutions
Tagging racks has never been easier. Customise your racking solutions to suit your needs.
Let's rethink what Air Products can do for you...

MDR-Certified Liquid Nitrogen
Storing human tissue samples? Your gas needs to be Medical Device regulation (MDR) certified - and we're the UK's only liquid nitrogen manufacturer to comply.
Learn more
Liquid Nitrogen Supply & Delivery
Whatever your needs, we offer a total cryogenic storage solution using MVE's wide range of liquid nitrogen storage vessels - widely regarded as the best available.
Learn more
Design Consultation
Setting up a new cryoroom or expanding an existing one, we provide a full design and installation service to make sure it works efficiently.
Learn moreOur products
At Air Products, we specialise in providing complete cryogenic storage solutions to perfectly suit your needs. That's why we work with MVE, one of the world's leading suppliers, to provide cryo storage to suit every user.

Liquid Nitrogen Freezers
We offer one of the largest selections of liquid nitrogen freezers in the world.

Industry applications

Cancer Research
Invitro Fertilisation (IVF)
Stem Cell Research
Vaccines

Cord Blood



Research Laboratories

Animal Husbandry
Looking to make changes to your cryo-room?
Industry applications
We collaborate with laboratories, pharmaceutical firms, fertility clinics, biotechnology companies,
biobanks, research centres, and universities
to drive advancements in the life sciences sector.
Explore market sectors we operate in to discover tailored solutions for your industry. Select your
relevant sector below to find the perfect products to meet your needs.
Invitro Fertilisation (IVF)
Learn moreCord Blood
Learn moreResearch Laboratories
Learn moreAnimal Husbandry
Learn moreStem Cell Research
Learn moreBlood Banks
Learn moreExperts in
cryogenic storage
From IVF to blood banks to research and training programmes, cryopreservation - keeping samples below -135°C to prevent the natural breakdown
of cells and keep sample characteristics intact - has become widely used.
Air Products has over 30 years` experience of helping hundreds of facilities from fertility labs to stem cell research,
so can offer you expert advice on what cry techniques and products will be best for your particular project or
application. Just contact our team today.
The right fit for you and your samples
From gas supply to laboratory design, installation, sample storage, training and ongoing maintenance and support, we're here to help you find the optimum cryogenic storage for your needs. Start by using this interactive selector guide to find the right options for you.
Arrange to speak to
an expert
Complete the form below and one of our experts will be in touch.
Download our
brochure

News

Air Products at Fertility Conference 2025
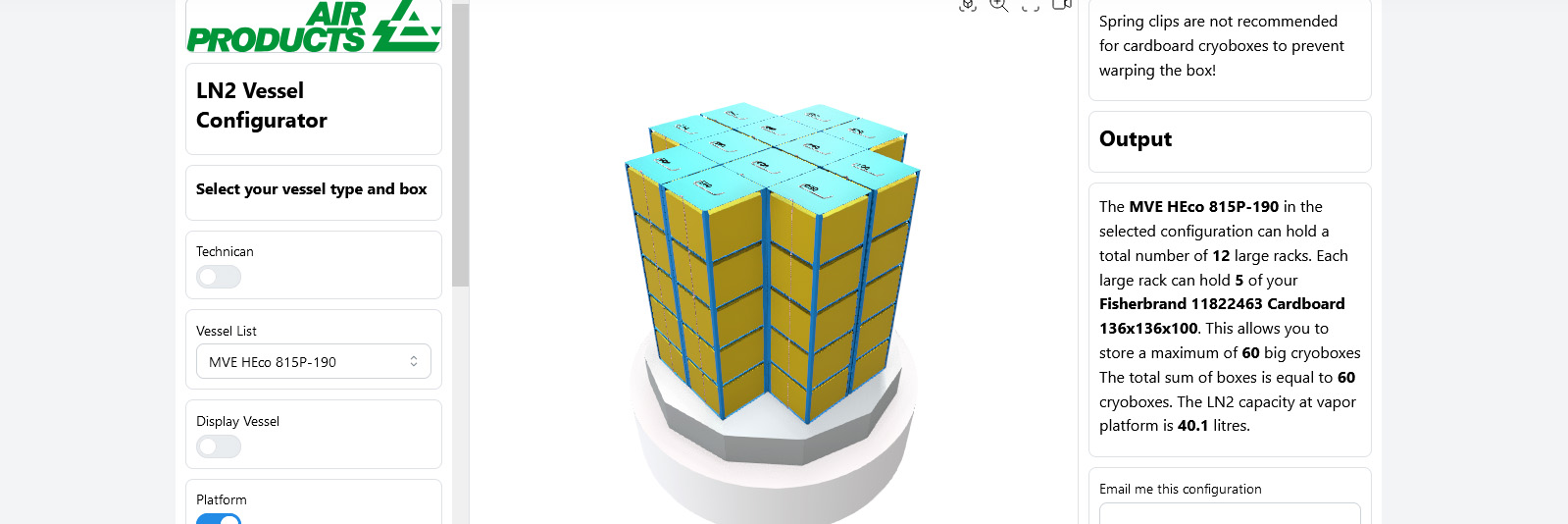
Racking Configurator

Air Products to Exhibit at Cell UK Congress 2024

Air Products to Exhibit at Lab Innovations 2024
Latest articles

Are you taking a risk with non-compliant biomedical equipment?
Read More
Disaster prevention: why it’s critical in cryopreservation
Read More
Planning for lab sustainability
Read More
Is your lab as safe as it should be?
Read More
Improve Your Labs Efficiency
Read More
Rethink your cryo-room for improved efficiency and sustainability
Read More
Anthony Nolan's stem cell registry aids blood cancer patients. With Air Products, they enhance cryopreservation to store cells, supporting life-saving treatments.
Read More
Discover expert solutions to optimize your fertility clinics cryo storage, maximizing space and efficiency.
Read More
Is your cryo-room consuming too much liquid nitrogen?
Read More
Are your samples as safe as they could be?
Read More
Long term cost efficiency in cryopreservation
Read More
How long can you store stem cells?
Read More
Are you taking a risk with non-compliant biomedical equipment?
Navigating Compliance: The Role of CE Marking & MDD/MDR in Biomedical Equipment
Read More
Disaster prevention: why it’s critical in cryopreservation
Disaster prevention and recovery with power outages or equipment failure
Read More
Planning for lab sustainability
How lab sustainability improves efficiency and reduces costs
Read More
Is your lab as safe as it should be?
Putting safety first in cryo-labs protects people and samples, boosts efficiency, and helps ensure compliance.
Read More

Rethink your cryo-room for improved efficiency and sustainability
How the location of your cryo-room leads to better efficiency and lower costs.
Read More
Anthony Nolan - The use of cryopreservation in life-saving research
Anthony Nolan's stem cell registry aids blood cancer patients.
Read More
Rethink your fertility clinics cryo storage space.
Discover expert solutions to optimize your fertility clinics cryo storage, maximizing space and efficiency.
Read More
Is your cryo-room consuming too much liquid nitrogen?
Get our expert opinion on how to reduce your cryo-rooms liquid nitrogen consumption. Saving you money and making your lab more efficient and sustainable.
Read More
Are your samples as safe as they could be?
Discover our expert solutions to mitigate the risk of your cryopreservation failing and to ensure the protection of your labs vital samples.
Read More
Long term cost efficiency in cryopreservation
Discover our advice and guidance on how to make your labs cryopreservation efficient and cost effective in the long term.
Read More
How long can you store stem cells?
While most cells in the body are differentiated and serve a specific function, stem cells are undifferentiated, meaning they can develop into many cell types that can serve numerous purposes. These cells are able to divide... indefinitely, and in the process, can either remain a stem cell or transform into a differentiated cell to aid the body.
Read More
Fertility sample storage changes
Anyone that works in the fertility or assisted reproduction industry can't have missed the recent headlines around the storage of fertility samples. Current storage limits are 10 years, after which a storage facility, if the owners haven't elected to donate them, will remove the samples and allow them to perish.
Read More
Frosty Fortunes: Prioritizing Quality
As you contemplate the acquisition of a cryogenic freezer, you may find yourself in a familiar dilemma. Drawn towards the most economical options, you question whether these freezers are actually good value for money.
Read More
Cryoroom Project Management
Managing a Cryoroom project for installation at a medical or scientific facility can be a daunting task with a range of potential obstacles to consider. Air Products Cryoroom Design and Project Management services are here to help you through this complex process.
Read More
How Energy Efficient Are Liquid Nitrogen Freezers?
In facilities such as blood banks, medical research centres and fertility clinics, the safe storage of biological samples is vital. Long term preservation of such fragile samples requires extremely low minimum temperatures of -135 degrees Celsius, alt the way down to -196 degrees Celsius.
Read More
How cryogenics have revolutionised animal breeding
The impact that human beings have had on the lives and development of other animals cannot be understated –while some species have been systematically bred for our purposes, others have been devastated by human colonisation through loss of habitat and hunting.
Read More